Lithium batteries power everything from smartphones to electric vehicles. They're compact, efficient, and reliable. But they can also catch fire. You've probably seen the news stories—a phone exploding, an e-bike bursting into flames, or an electric car fire that took hours to put out. These incidents raise an obvious question: why do lithium batteries catch fire in the first place?
We're breaking down the science behind these fires, the conditions that trigger them, and what you can do to stay safe. Whether you're using lithium coin cells in your watch or rechargeable batteries in your tools, understanding the risks helps you make smarter choices.
What Is Thermal Runaway?
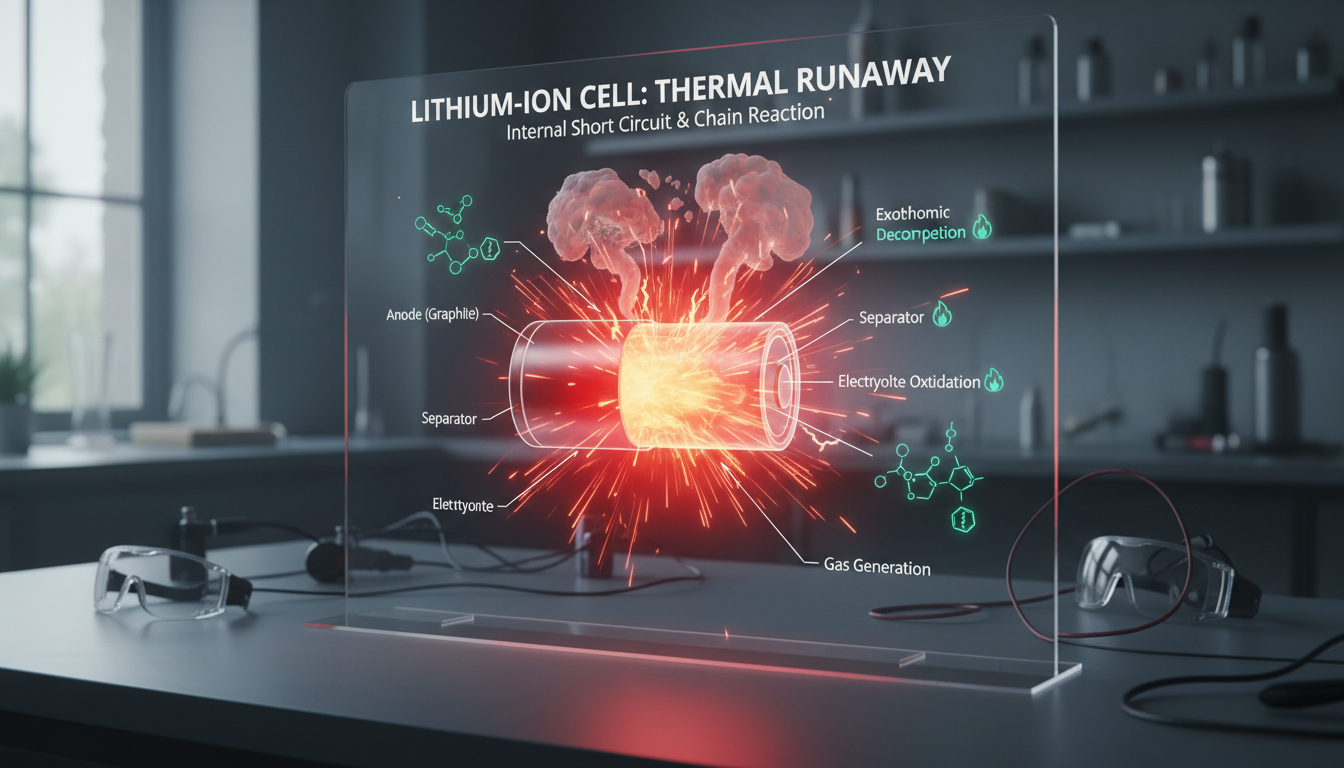
Thermal runaway is a dangerous and self-sustaining reaction in lithium-ion batteries that occurs when heat generation exceeds the battery's ability to dissipate it. Think of it as a chain reaction. Once it starts, it's almost impossible to stop.
This chain reaction creates extremely high temperatures (around 752 degrees Fahrenheit / 400 degrees Celsius). At those temperatures, the battery's internal components break down. The materials in a lithium-ion battery's separator and electrolyte blend are combustible. When these batteries experience thermal runaway, results can include overheating, flaming, and sometimes an explosion.
The stored energy releases all at once, often with visible flames and toxic smoke. One report states only 15 seconds between the first sign of smoke and the windows of a house being blown out. That's how fast things can go wrong.
Physical Damage and Manufacturing Defects

One of the main causes of lithium-ion battery failures leading to Thermal Runaway and, ultimately, fires or explosions is excessive physical stress. Drop your phone? Crush a battery pack? That internal damage might not show on the outside, but it can create problems inside the cell.
If a battery is subjected to high-pressure levels, such as being punctured or crushed, it can become unstable and catch fire. The separator—a thin layer that keeps the positive and negative sides apart—can tear. When that happens, you get an internal short circuit.
Manufacturing defects play a role too. Most serious incidents are not caused by normal operation, but by poor-quality products, lack of certification, improper charging, physical damage, or unsafe storage. Low-quality or counterfeit batteries skip safety features. They might lack proper battery management systems or use substandard materials that fail under stress.
Overcharging and Charging Issues
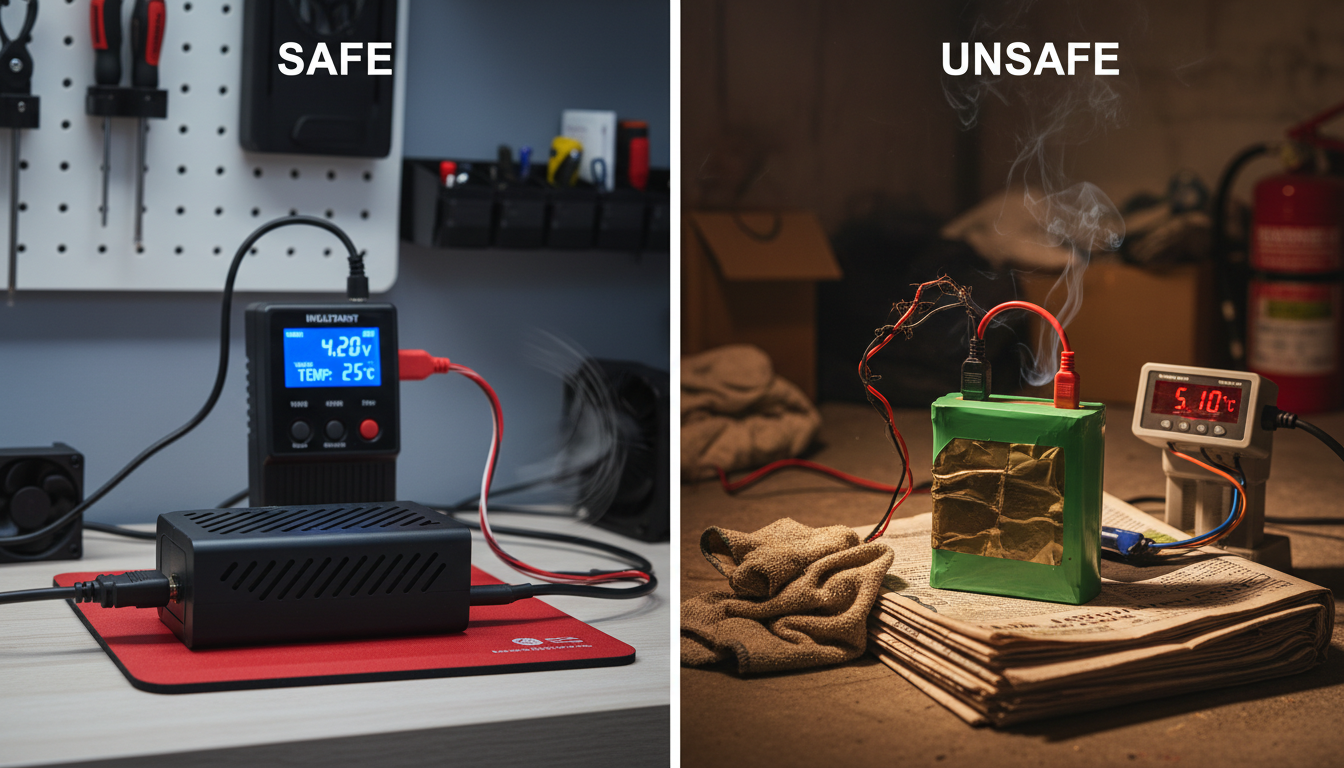
Leaving your device plugged in overnight might seem harmless, but overcharging is a real fire risk. When a lithium battery is overcharged, immediate and long-term damage can occur: Heat Build-Up: Overcharging generates excess heat, potentially causing swelling, breakdown of internal chemistry, or even thermal runaway (fire risk).
When lithium-ion batteries are charged too quickly, chemical reactions can produce very sharp lithium needles called dendrites on the battery's anode. Eventually, they penetrate the separator and reach the other electrode, short-circuiting the battery internally. These dendrites are like tiny metal bridges that create paths for unwanted electrical flow.
Using the wrong charger makes things worse. If a battery is charged using a charger that is incompatible with the battery, or if it is charged using a damaged or faulty charger, it can become unstable and lead to Thermal Runaway. Cheap aftermarket chargers often lack the voltage regulation needed to safely charge lithium batteries.
Temperature Extremes
Batteries don't like extreme temperatures—hot or cold. Another common cause of lithium-ion battery fires is exposure to high temperatures. These batteries are designed to operate within a specific temperature range.
High ambient temperatures are one of the most important risk factors. Heat accelerates chemical reactions inside the battery, raises internal pressure, and reduces the safety margin of internal components. Leaving batteries in hot cars, storing them near heat sources, or using them in direct sunlight all increase fire risk.
But cold isn't safe either. Overcharging is more likely to happen at low temperatures because the upper voltage limit is more simply exceeded due to the higher internal resistance. Chemical reactions slow down in the cold, which can confuse charging systems and lead to voltage spikes.
Battery Age and Degradation
Lithium-ion batteries degrade over time due to charge and discharge cycles. Every time you charge and drain a battery, its internal structure changes slightly. After hundreds or thousands of cycles, the damage adds up.
It is typical for rechargeable batteries to last two to three years or up to 500 discharge/charge cycles. Some signs of age include the battery discharging more quickly than is typical, getting hot or swelling, or losing the charge when not in use.
Old batteries lose their ability to handle heat. Their internal resistance increases, which means they generate more heat during normal use. This makes them more vulnerable to thermal runaway, even during routine charging.
How to Prevent Battery Fires
Prevention starts with smart choices. For users, the most effective prevention starts at the point of purchase. Choosing batteries from reputable brands and established manufacturers, rather than focusing only on the lowest price, greatly reduces safety risk. Look for certified batteries with proper safety testing.
Stop using lithium-ion batteries if you notice an odor, change in color, too much heat, change in shape, leaking or odd noises. These warning signs mean something's wrong inside the battery.
Don't charge the batteries overnight or when you can't keep an eye on them. It's also important to plug your devices directly into a wall socket using the charger that came with them. Avoid extension cords and power strips when charging high-capacity batteries.
Store batteries in cool, dry places. Store spare lithium-ion batteries away from anything that can burn. Don't put lithium-ion batteries in direct sunlight or keep them in hot cars. This is a fire risk.
Conclusion
Lithium batteries catch fire when thermal runaway occurs—a self-feeding reaction triggered by physical damage, overcharging, temperature extremes, manufacturing defects, or battery age. The chemistry inside these batteries is inherently volatile under the wrong conditions. Understanding these triggers helps you use batteries more safely and recognize when one needs to be replaced. While battery fires remain relatively rare compared to the billions of batteries in use, the consequences can be severe. Choose quality products, follow charging guidelines, watch for warning signs, and store batteries properly. These simple steps reduce your risk and help you get the most out of your battery-powered devices.
FAQs
Can lithium batteries catch fire when not in use?
Yes, though it's less common. There have been documented cases of batteries igniting during storage due to internal defects, prior damage, or long-term degradation. Fully discharging a lithium battery is not a safe solution either, as deep discharge can destabilize the cell chemistry and damage protective circuits, increasing long-term safety risks. Internal short circuits can develop over time, especially in damaged or defective batteries.
Are some types of lithium batteries safer than others?
Yes. LiFePO4 (lithium iron phosphate) can still be damaged by overcharging (and can still swell, vent, or fail), but it is much less likely to enter thermal runaway or catch fire compared to NMC. The iron-phosphate chemistry is less flammable and more resistant to overheating. However, all lithium batteries require proper handling and protection systems.
What should I do if I see a lithium battery fire?
The best advice when encountering a fire from a lithium-ion battery is to leave the area and call 9-1-1. These types of fires burn very hot, can spread quickly, and can even cause explosions. Because these fires are caused by flammable liquids inside the battery, water or foam extinguishers will not always put them out. Don't try to fight large battery fires yourself.
How can I tell if my battery is about to fail?
Watch for physical changes and performance issues. Look for swelling, excessive heat, chemical smells, leaking, rapid self-discharge, or fluctuating voltage. If your battery shows any of these symptoms, stop using it immediately and dispose of it properly at a battery recycling location.
Do battery management systems prevent all fires?
Battery management systems can monitor vital battery parameters, such as the state of charge, internal pressure and the temperature of the cells in the battery pack. Battery management systems can diagnose conditions within the battery pack and make autonomous decisions to shut off batteries with hot spots, or to alter the load distribution so that any individual battery does not get too hot. But they're not foolproof—they can fail or be overwhelmed by extreme abuse conditions.



















