Ever wondered what makes your smartphone, laptop, or electric vehicle run for hours on end? Lithium batteries power our modern world, but most people don't know what's actually inside them. We'll break down the key materials and components that make these energy powerhouses work.
Lithium batteries aren't just a single blob of material—they're carefully engineered systems with multiple components working together. From the metals that store energy to the separators that keep everything safe, each part plays a specific role. And here's the thing: not all lithium batteries are created equal. Different materials create different battery types, each with its own strengths.
Core Components of Lithium Batteries
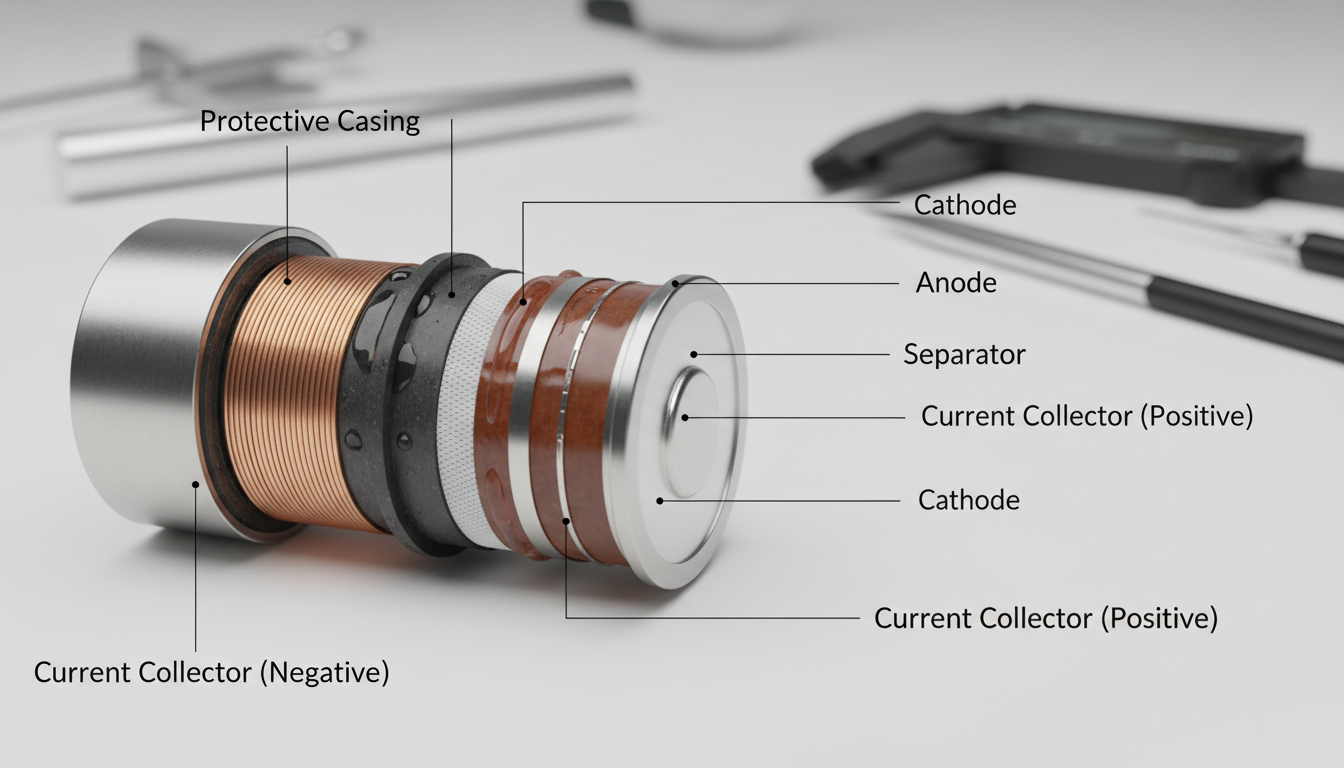
Lithium batteries contain six main parts that work together to store and deliver power. The core battery components include the anode, cathode, electrolyte, separator, current collectors, and casing. Think of it like a well-organized team where each player has a specific job.
The anode serves as the negative electrode. It's made up of a porous carbon-based material, typically graphite, and stores lithium ions when you charge your battery. During discharge, those ions move out to create power.
The cathode is the positive electrode and acts as the source of lithium ions. It's generally one of three materials: a layered oxide (such as lithium cobalt oxide), a polyanion (such as lithium iron phosphate) or a spinel (such as lithium manganese oxide). The cathode material you choose determines how much energy the battery holds and how long it lasts.
The Electrolyte: Ion Highway

The electrolyte is formed of salts, solvents and additives, and serves as the conduit of lithium ions between the cathode and anode. Without it, your battery would be dead on arrival.
The non-aqueous electrolyte is typically a mixture of organic carbonates such as ethylene carbonate and propylene carbonate containing complexes of lithium ions. Most batteries use liquid electrolytes with lithium salts like LiPF6 or LiBF4 dissolved in organic solvents.
But there's a catch. Liquid electrolytes can break down over time, reducing battery performance. Heat and deep discharges speed up this process. That's why researchers are working on solid-state electrolytes, which promise better safety and longer life.
Separator and Current Collectors

The separator is a thin, porous membrane that separates the anode and cathode and prevents them from touching. If those two electrodes made contact, you'd have a short circuit—and potentially a fire. Most separators are made from plastics like polyethylene (PE) or polypropylene (PP).
Current collectors do exactly what their name suggests—they collect the electrical current. Common metals used for current collectors include aluminum for the cathode and copper for the anode. These materials are picked for their conductivity and durability.
Raw Materials: What Goes Into Making These Batteries
Before batteries are assembled, manufacturers need specific raw materials. Critical raw materials used in manufacturing lithium-ion batteries include lithium, graphite, cobalt, and manganese. Each plays a different role in battery performance.
Graphite dominates the anode side. Graphite is the most common anode material because it conducts electricity well, costs less, and is stable. Some newer batteries add silicon to graphite for extra storage capacity, though this creates challenges with battery expansion during charging.
On the cathode side, lithium combines with various metals. Cobalt, nickel, manganese, and iron are common choices. Each metal brings different characteristics—cobalt offers high energy density, iron phosphate provides better safety, and manganese adds thermal stability.
Different Battery Chemistries Explained
Not all lithium batteries use the same chemical makeup. The chemistry you pick depends on what you need the battery to do. Need safety and long life? Lithium iron phosphate (LFP) is your answer. Looking for maximum energy in a small package? Lithium cobalt oxide (LCO) might work better.
Lithium cobalt oxide has the chemical symbols LiCoO2 and the abbreviation LCO. You'll find LCO batteries in smartphones and laptops because they pack a lot of power into tight spaces. But they're not ideal for applications needing extended lifespans.
Lithium nickel manganese cobalt oxide (NMC) batteries blend three metals for balanced performance. The three active materials of nickel, manganese and cobalt can easily be blended to suit a wide range of applications for automotive and energy storage systems. Electric vehicles often use NMC chemistry because it offers both power and range.
At Voniko Batteries, we offer various battery types for different needs. Our lithium battery products and rechargeables are designed with quality materials to deliver reliable performance.
Safety Features and Battery Casing
Safety isn't an afterthought in battery design—it's built into every component. The lithium-ion battery casing is the protective outer structure that holds the internal components of a lithium-ion battery, ensuring the safety of the battery and its surroundings by containing and insulating the potentially volatile materials within.
Battery cases are typically made from durable, heat-resistant materials like plastics, metals (aluminum or steel), or composite materials. The choice depends on the application. A battery for your phone needs different protection than one for an electric car.
Many batteries also include additional safety features like protection circuits to prevent overcharging and thermal management systems. If you're interested in battery safety, check out our guide on how to put out a lithium-ion battery fire.
How These Materials Work Together
A lithium-ion battery operates by shuttling lithium ions back and forth between the anode and cathode through the electrolyte, with the flow of electrons controlled by the external circuit, generating electrical energy during discharge and allowing for the recharging of the battery during the charging phase.
When you plug in your device to charge, lithium ions move from the cathode through the electrolyte to the anode, where they're stored. When you use your device, the process reverses—ions flow back to the cathode, creating electrical current that powers your gadget.
The separator keeps this process safe by blocking direct electron flow while allowing ions to pass through. Current collectors gather the electrons and channel them through your device. It's a synchronized dance of chemistry and physics.
Conclusion
Lithium batteries contain carefully selected materials that work together to store and deliver energy. The main components—anode (typically graphite), cathode (lithium metal oxides), electrolyte (lithium salts in organic solvents), separator (plastic membrane), current collectors (copper and aluminum), and casing—each serve specific functions. Different cathode materials create different battery chemistries with unique advantages. Lithium cobalt oxide offers high energy density for consumer electronics, while lithium iron phosphate provides safety and longevity for industrial applications. The critical raw materials include lithium, graphite, cobalt, nickel, and manganese. These batteries work by moving lithium ions between electrodes, creating electrical current that powers everything from smartphones to electric vehicles.
FAQs
What are the main materials inside lithium batteries?
Lithium batteries contain graphite for the anode, lithium metal oxides (like cobalt oxide or iron phosphate) for the cathode, lithium salts dissolved in organic carbonates for the electrolyte, and plastic membranes for the separator. Current collectors made from copper and aluminum complete the main materials. Each material is chosen for specific properties like conductivity, stability, and energy storage capacity.
What's the difference between lithium-ion and lithium metal batteries?
Lithium-ion batteries use lithium compounds in their electrodes and move lithium ions during charging and discharging. Lithium metal batteries use pure metallic lithium at the anode. Lithium-ion batteries are rechargeable and safer for consumer use, while lithium metal batteries (like coin cells) are often single-use but offer higher energy density. The lithium-ion design prevents dangerous dendrite formation that plagued early lithium metal batteries.
Why do different lithium batteries have different lifespans?
The cathode material determines much of a battery's lifespan. Lithium iron phosphate batteries can last 2,000-3,000 charge cycles, while lithium cobalt oxide batteries typically last 500-1,000 cycles. The chemistry affects how well the battery structure holds up during repeated charging and discharging. Temperature, charging habits, and discharge depth also impact longevity.
Are lithium batteries recyclable?
Yes, lithium batteries contain valuable materials that can be recovered and reused. Recycling processes can recover 95% of lithium along with cobalt, nickel, and other metals. These recovered materials can be processed into new battery components, reducing the need for mining and lowering environmental impact. Proper disposal and recycling also prevent hazardous materials from ending up in landfills.
Can I use alkaline batteries instead of lithium batteries?
It depends on your device. Lithium batteries offer higher energy density, longer shelf life, and better performance in extreme temperatures compared to alkaline batteries. They weigh less and can store 150 watt-hours per kilogram versus 25 watt-hours for alkaline. However, alkaline batteries cost less upfront and work fine for low-drain devices like remote controls. High-drain devices like digital cameras perform better with lithium.




















